
The extremely low melting and boiling points result from weak forces of attraction between the molecules. The existence of these weak intermolecular forces is also revealed by the fact that, when hydrogen gas expands from high to low pressure at room temperature, its temperature rises, whereas the temperature of most other gases falls. According to thermodynamic principles, this implies that repulsive forces exceed attractive forces between hydrogen molecules at room temperature—otherwise, the expansion would cool the hydrogen. In fact, at −68.6° C attractive forces predominate, and hydrogen, therefore, cools upon being allowed to expand below that temperature. The cooling effect becomes so pronounced at temperatures below that of liquid nitrogen (−196° C) that the effect is utilized to achieve the liquefaction temperature of hydrogen gas itself. A molecular form called protonated molecular hydrogen (H+3) is found in the interstellar medium, where it is generated by ionization of molecular hydrogen from cosmic rays.
Hydrogen
Ignition of leaking hydrogen is widely assumed to be the cause, but later investigations pointed to the ignition of the aluminized fabric coating by static electricity. But the damage to hydrogen's reputation as a lifting gas was already done and commercial hydrogen airship travel ceased. Hydrogen is still used, in preference to non-flammable but more expensive helium, as a lifting gas for weather balloons. In Metelko alphabet, the phoneme was written by two different letters whether it was pronounced as velar /x/ or glottal /h/, a distinction irrelevant to nowadays standard and the distinction was also not used by all writers. Phoneme /h/ was written with 〈h〉, while /x/ was written with a yet to be encoded character .
Biological reactions
See the Silesian language article on Wikipedia for more, and h for development of the glyph itself. In Irish, ⟨h⟩ is not considered an independent letter, except for a very few non-native words, however ⟨h⟩ placed after a consonant is known as a "séimhiú" and indicates lenition of that consonant; ⟨h⟩ began to replace the original form of a séimhiú, a dot placed above the consonant, after the introduction of typewriters. Its most important uses are in the digraphs 'ch' /k/ and 'gh' /ɡ/, as well as to differentiate the spellings of certain short words that are homophones, for example some present tense forms of the verb avere ('to have') (such as hanno, 'they have', vs. anno, 'year'), and in short interjections (oh, ehi).
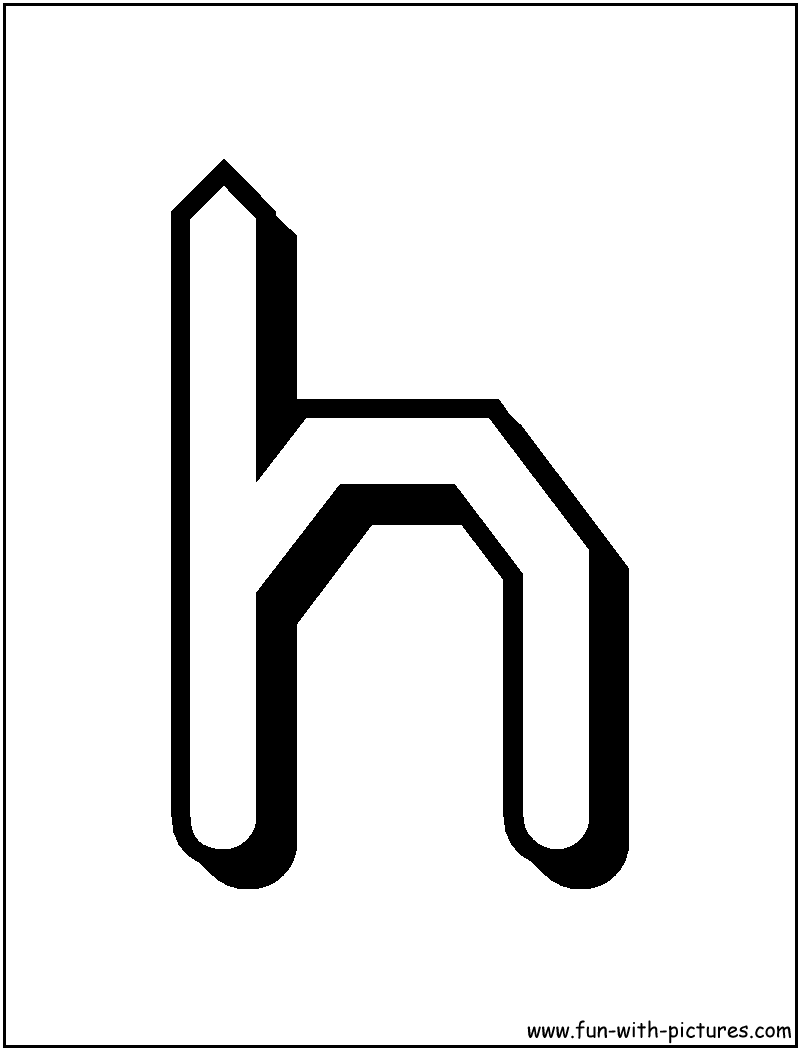
Alternative forms
In the quantum mechanical treatment, the electron in a ground state hydrogen atom has no angular momentum at all—illustrating how the "planetary orbit" differs from electron motion. In the early Greek alphabets a form with three horizontal bars and the simpler form H were both widely distributed. In Etruscan the prevailing form was similar to the early Greek form, and the same or a similar form occurs in very early Latin inscriptions, but the form H came into general use in Latin, either from the Chalcidic Greek alphabet of Cumae or from some other source. The cursive Latin form resembled a stylized version of the modern minuscule h, as did the uncial form.
See the history of Polish orthography article on Wikipedia for more, and h for development of the glyph itself. See the Kashubian alphabet article on Wikipedia for more, and h for development of the glyph itself. In Basque, during the 20th century it was not used in the orthography of the Basque dialects in Spain but it marked an aspiration in the North-Eastern dialects.

During the standardization of Basque in the 1970s, the compromise was reached that h would be accepted if it were the first consonant in a syllable. Hence, herri ("people") and etorri ("to come") were accepted instead of erri (Biscayan) and ethorri (Souletin). For the dialects lacking the aspiration, this meant a complication added to the standardized spelling.
NCAE slams new H-2A rule - The Packer
NCAE slams new H-2A rule.
Posted: Wed, 01 May 2024 14:24:20 GMT [source]
Role in quantum theory
Note that it represents the sound of IPA [x] (like German machen, ach), not (as in most other alphabets based on the Latin script) the sound of IPA [h]. In English the initial h is pronounced in words of Germanic origin (e.g., hunt, hook); in some words of Romance origin, the h remains unpronounced (e.g., heir, honour), but in others it has been restored (e.g., humble, humour). The initial h often disappears in unaccented syllables (e.g., “What did he say?”). 'H' is also used in many spelling systems in digraphs and trigraphs, such as 'ch', which represents /tʃ/ in Spanish, Galician, and Old Portuguese; /ʃ/ in French and modern Portuguese; /k/ in Italian and French. Oxidation of hydrogen removes its electron and gives H+, which contains no electrons and a nucleus which is usually composed of one proton. Under the Brønsted–Lowry acid–base theory, acids are proton donors, while bases are proton acceptors.
It was formerly common for an rather than a to be used as the indefinite article before a word beginning with /h/ in an unstressed syllable, as in "an historian", but use of a is now more usual (see English articles § Indefinite article). Hydrogen is mainly produced by steam methane reforming (SMR), the reaction of water and methane.[103][104] [105] Thus, at high temperatures (1000–1400 K, 700–1100 °C or 1300–2000 °F), steam (water vapor) reacts with methane to yield carbon monoxide and H2. But whencesoever this stinking smoak proceeded, so inflammable it was, that upon the approach of a lighted candle to it, it would readily enough take fire, and burn with a blewish and somewhat greenish flame at the mouth of the viol for a good while together; and that, though with little light, yet with more strength than one would easily suspect. In the alphabets used to write the East Ionic dialect of Greek the letter became superfluous as a result of the disappearance of the aspirate which it represented in that dialect.
Electron energy levels
It was accordingly put to a new use to indicate the open long e which had arisen through alteration of the primitive Greek long a. In a few inscriptions from Thera, Naxos, and several other localities the letter was used with syllabic value; that is, it included he, thus showing its old consonantal and its new vocalic value at the same time. Eventually, as a result of the spread of the Ionic alphabet, its use for the long vowel e or η became general throughout Greece, while its consonantal value as the aspirate h passed from the western Greek alphabets into the Etruscan alphabets and then into the Latin and other alphabets of ancient Italy. In the Romance languages the sound has largely disappeared, but the letter is still extensively used, partly with only etymological value, (e.g., French homme), partly with fancied etymological value (e.g., French haut from Latin altus, with h through the influence of hoh, the Old High German word of the same meaning), partly with special orthographical functions.
The early contemporary variant was first found in the dialect of lsien tal-bliet (“tongues of the cities”, referring to the cities around the Grand Harbour according to Vassalli) which eventually superceded the increasingly archaic [h] sound in the neighborhing areas.
Recycling and 4-H collaboration continues into a third year - Idaho County Free Press
Recycling and 4-H collaboration continues into a third year.
Posted: Wed, 01 May 2024 11:00:00 GMT [source]
The gas, however, was confused with other flammable gases, such as hydrocarbons and carbon monoxide. In 1766 Henry Cavendish, English chemist and physicist, showed that hydrogen, then called flammable air, phlogiston, or the flammable principle, was distinct from other combustible gases because of its density and the amount of it that evolved from a given amount of acid and metal. In 1781 Cavendish confirmed previous observations that water was formed when hydrogen was burned, and Antoine-Laurent Lavoisier, the father of modern chemistry, coined the French word hydrogène from which the English form is derived. In 1929 Karl Friedrich Bonhoeffer, a German physical chemist, and Paul Harteck, an Austrian chemist, on the basis of earlier theoretical work, showed that ordinary hydrogen is a mixture of two kinds of molecules, ortho-hydrogen and para-hydrogen. Because of the simple structure of hydrogen, its properties can be theoretically calculated relatively easily.
In lithium aluminium hydride, the [AlH4]− anion carries hydridic centers firmly attached to the Al(III). Throughout the universe, hydrogen is mostly found in the atomic and plasma states, with properties quite distinct from those of molecular hydrogen. As a plasma, hydrogen's electron and proton are not bound together, resulting in very high electrical conductivity and high emissivity (producing the light from the Sun and other stars). The charged particles are highly influenced by magnetic and electric fields.
This is because ⟨j⟩ and ⟨v⟩ used to be considered variants of ⟨i⟩ and ⟨u⟩ respectively. ⟨h⟩ also appears in the digraph ⟨ch⟩, which represents /tʃ/ in Spanish and northern Portugal, and /ʃ/ in varieties that have merged both sounds (the latter originally represented by ⟨x⟩ instead), such as most of the Portuguese language and some Spanish dialects, prominently Chilean Spanish. The Table lists the important properties of molecular hydrogen, H2.
Historically, hydrogen gas was first produced artificially in the early 16th century through the reaction of acids with metals. Henry Cavendish, between 1766 and 1781, identified hydrogen gas as a distinct substance[16] and discovered its property of producing water when burned—hence its name derived from the Greek "water-former". Although hydrogen is the most abundant element in the universe (three times as abundant as helium, the next most widely occurring element), it makes up only about 0.14 percent of Earth’s crust by weight. It occurs, however, in vast quantities as part of the water in oceans, ice packs, rivers, lakes, and the atmosphere. As part of innumerable carbon compounds, hydrogen is present in all animal and vegetable tissue and in petroleum.

No comments:
Post a Comment